Biophysical studies on knotted tRNA methyltransferase (TrmH) and clarification of its physiological function
A. Ochi, K. Makabe, K. Kuwajima, and H. Hori,
“Flexible recognition of the tRNA G18 methylation target site by TrmH methyltransferase through first binding and induced fit processes”
J. Biol. Chem. 285, 9018-9029 (2010)
S. Jockel, G. Nees, R. Sommer, Y. Zhao, D. Cherkasov, H. Hori, G. Ehm, M. Schnare, M. Nain, A. Kaufmann, and S. Bauer, “The 2’-O-methylation
status of a single buanosine contorls transfer RNA mediated Toll-like recptor
7 activation or inhibition.”
J. Exp. Med. 209, 235-241 (2012)

Our structure-based biophysical study clarified the tRNA recognition mechanism
of knotted tRNA methyltransferase.
On the other hand, our physiological study of this enzyme was selected as a Research Highlight in Nature Reviews.
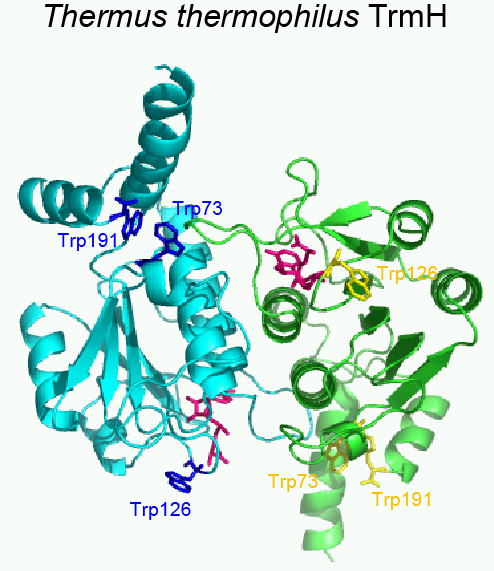
A. Ochi , K. Makabe , R. Yamagami , A. Hirata , R. Sakaguchi , Y. M. Hou , K. Watanabe , O. Nureki , K. Kuwajima , and H. Hori ,
“The Catalytic Domain of Topological Knot tRNA Methyltransferase (TrmH)
Discriminates between Substrate tRNA and Nonsubstrate tRNA via an Induced-fit
Process.”
J. Biol. Chem. 288, 25562-25574 (2013)